#1. A Redox Health Model for Cardiovascular Disease
“…The common feature of all risk factors of cardiovascular diseases is imbalance between pro- and anti-oxidative factors in the organism with an increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS are key players in normal cardiovascular physiology and signaling, but their increased levels lead to cellular injury and death (necrosis or apoptosis) through direct effects (lipid peroxidation) or indirect effects (activation of the redox signaling pathways).[49]” —M. Barančík, et al. in Physiological Research
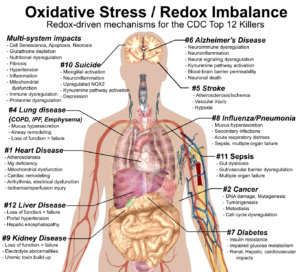
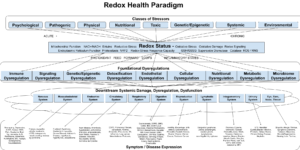
Cardiovascular disease is the number one killer in the US and globally. Indeed, when you look at the average workplace wellness program, it focuses almost entirely on heart-disease-related biomarkers—cholesterol, triglycerides, BMI, blood pressure, waist-hip ratio, etc. A redox health paradigm both critiques our current approach to heart disease and provides a more robust understanding of why it happens.
Weaknesses of the Current Paradigm: The Lipid Hypothesis No Longer Cuts It
The bedrock of our current approach to preventing heart disease is the “lipid hypothesis,” which posits that lowering cholesterol will lower the incidence of heart disease. While the hypothesis garnered some early well-deserved criticism, it ultimately carried the day and became medical dogma that continues to this day. So what’s the problem?
We’ve made high cholesterol into a disease, and it’s not. Cholesterol is a biomarker. The problem with turning a biomarker into a disease is that you may not understand the role the biomarker is playing in the disease. In the case of cholesterol, by focusing on lowering lipids we’re ignoring things that are actually causal in heart disease: oxidative stress and inflammation.
Statins Are Imperfect Drugs
For several decades, the linchpin therapy for lowering cholesterol has been statins like Lipitor, Zocor, and Crestor. As recently as 2012, overly exuberant US health officials were promoting the idea of adding statins to the water supply! In recent years, however, dissenting voices have pointed out both worrisome side effects and studies showing that statins just aren’t that effective.
One approach I found particularly enlightening comes from the esoteric world of medical statistics—an approach to therapeutic assessment called “number needed to treat” or NNT (and conversely, “number needed to harm”). Put simply, NNT counts the number of patients you’d need to treat before one of them was helped. Paired with that is a count for the number of patients you’d need to treat before one of them was harmed.[50]
For statins prescribed over a five-year period for heart disease prevention (without known heart disease), the NNT results fall somewhere between underwhelming and alarming:
| Benefits in NNT | Harms in NNT | |
| • None were helped (life saved)• 1 in 104 were helped (preventing heart attack)• 1 in 154 were helped (preventing stroke) | • 1 in 50 were harmed (developed diabetes)• 1 in 10 were harmed (muscle damage) |
This is useful information for understanding the risk/benefit of a particular therapy, and for primary prevention of heart disease, statins have some significant downsides—harms. More detailed research into the behaviors of statins shows that they reduce CoQ10 levels, interfere with the recycling of NAD+ and in general contribute to mitochondrial dysfunction which can lead to myopathies and diabetes.
Population studies tend to gloss over side effects, and even the NNT approach gives the impression that these side effects are impacting one in ten for muscle damage and one in fifty for diabetes. However, there is every reason to believe that these dynamics are affecting everyone who takes statins. They just don’t rise to the level of a clinical diagnosis or a patient complaint to their physician. The reason you should care about statin side effects is that to do otherwise means that you and your doctor are not likely to try to either mitigate the downsides or find safer, more effective therapies.
Redox Health and the Mechanisms of Heart Disease
A redox health paradigm gives us a framework for understanding the mechanisms of heart disease, and much progress has been made in understanding the individual pathologies that drive chronic heart disease. As you might expect, oxidative stress and inflammation play a central role.
The inflammatory hypothesis of heart disease was recently (2018) confirmed in the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS), where an anti-inflammatory drug reduced the number of recurrent major cardiac events in a cohort of participants with prior heart attacks. The drug had enough downsides that you won’t be getting a prescription for canakinumab as a heart therapy, but the consensus was still that the inflammatory hypothesis of heart disease had been proven—a significant and little-noticed milestone in cardiovascular research.
At a very high level, prevention and treatment of cardiovascular disease should be concerned with the following:
- The health of your vasculature—veins, blood vessels, arteries.
- The electrical function of the heart—is it beating properly? Prone to abnormal rhythms and disruptions?
- The mechanical function and health of the heart and its component parts—valves, chambers, vessels, and tissues.
The details of how oxidative stress impacts these functions and leads to cardiovascular disease are complex, but we can still make useful generalizations.
The Stressors
The list of stressors has not changed dramatically from the list of risk factors in the current Western medical paradigm:
What has changed is the understanding that these and other risk factors contribute to our overall stress burden and can lead to redox imbalance, reduced stress resilience, and persistent inflammatory states (feedforward loops) that lead to cardiovascular disease, hypertension, and health events like arrhythmias, heart attack, sudden cardiac death, and stroke.
- Lack of sleep and disruption of circadian rhythms (e.g., from shift work)
- Lack of exercise
- Smoking
- Air pollution
- Overconsumption of processed carbs and sugar
- Overconsumption of omega-6 oils and trans fats
- Obesity and overeating
- Low magnesium and vitamin D status
- Low nitric oxide availability in the circulatory system
- Calcium loading in the cell
The Mechanisms
Rather than rehashing the role of various stressors, I want to focus your attention on the most important downstream effects of those stressors and the role they play in the onset and progression of cardiovascular disease. Unless you are reading primary medical research, almost none of them will be on your radar, and most likely will not have come up in conversations with your doctor.
Mechanism 1: Low Intracellular Magnesium Levels, Low Reduced Glutathione (GSH) Levels, Elevated Oxidative Stress
There’s reasonably good evidence that this is an early feedforward loop that contributes to cardiovascular disease. Magnesium is a cofactor in glutathione recycling, so low magnesium levels disrupt glutathione recycling, resulting in lower reduced glutathione levels. Magnesium is expelled from cells when under stress, so low glutathione status contributes to elevated oxidative stress. In this pro-oxidant state, lots of problematic things happen:
- Lipids, carbohydrates, proteins, and DNA get oxidized.
- Some of these oxidative byproducts, like peroxynitrite, are toxic and will cause additional oxidative stress, reduced vascular nitric oxide (NO) availability, and injury to arteries and blood vessels. It’s this injury, along with dysregulated NO, that contributes to atherosclerosis and narrowed, stiffened arteries.
- Redox signaling activates the immune system via the NF-κB pathway to assist in cleaning up the oxidative byproducts and other damage, and potentially to fight anything pathogenic that might be involved in creating this oxidative mess. Remember, since cells produce reactive oxygen and nitrogen species in response to virtually all stressors, the body is programmed to view elevated ROS/RNS levels as a general call to arms.
- An activated immune system and oxidative stress contribute to gut and vascular permeability, which allow bacteria and bacterial byproducts to pass into the bloodstream, where they cause immune system activation, systemic inflammation, and remote infections (e.g., bladder and urinary tract infections).
- Redox signaling activates the HPA axis and keeps the sympathetic nervous system activated. This results in an anabolic/catabolic imbalance, meaning that the forces that break down the body under stress (catabolic) predominate, versus the forces that rebuild the body after stress (anabolic).
- DNA damage stimulates the production of PARP, a family of proteins involved in DNA repair. While this may be useful and necessary, PARP production depletes NAD+ levels which contributes to impaired energy production and additional elevated oxidative stress, like a four-cylinder engine running on three cylinders.
- Mitochondrial dysfunction plays a central role in heart failure and heart attack, as well as obesity, metabolic syndrome, diabetes, and other diseases.
- Lowered magnesium disrupts the calcium–magnesium balance and can cause calcium loading in the cell, creating additional cellular stress, pathological signaling, damage, and cell death.
- Lowered magnesium levels can dysregulate sodium, potassium and phosphorus levels due to magnesium’s regulatory functions, particularly with parathyroid production and on parathyroid receptors.[51]
Mechanism 2: Lipopolysaccharide, aka Endotoxin
Lipopolysaccharide is a toxin found in the cell walls of gram-negative bacteria. When the intestinal barrier is permeable, lipopolysaccharide can pass into the bloodstream, where it powerfully activates the immune system and causes systemic inflammation and damage to the heart and kidneys (among other things). Injecting rats with lipopolysaccharide is a standard method of inducing depression and kidney / cardiac injury.
Mechanism 3: Peroxynitrite
One of the downstream effects of elevated superoxide levels is its propensity to bind with nitric oxide (NO) in the bloodstream to form peroxynitrite, a highly toxic compound that contributes to circulatory damage and inflammation. In addition to its role in damage and inflammation, peroxynitrite also reduces the availability of nitric oxide, which is an important redox signaling molecule for maintaining vascular health and homeostasis, contributing to vascular injury, constriction, stiffness, and atherosclerosis, all hallmarks of cardiovascular disease.
Mechanism 4: NLRP3 Inflammasome Activation
Inflammasomes are structures located inside the cell that are responsible for the production and maturation of signaling proteins known as cytokines. Cytokines have a variety of responsibilities, many of them pro-inflammatory. The NLRP3 inflammasome is the most studied inflammasome and is emerging as an important pathology in cardiovascular disease. Activation of the NLRP3 inflammasome is both initiated and sustained by oxidative stress.
Mechanism 5: Chronic Low-Grade Infection
Chronic low-grade infection (bacterial, viral, and fungal) is seriously underappreciated as a source of oxidative stress, immune system activation, and inflammation. While an acute infection may trigger a cardiac event due to elevated oxidative stress from the infection and your immune system’s response to it, chronic low-grade infection can lead to chronic, systemic, low-grade inflammation. This inflammation drives damage to your arteries and heart tissues. Your dentist will be happy to remind you that poor oral hygiene is one of the most common sources of chronic low-grade infection.
Mechanism 6: Mitochondrial Dysfunction
The heart is a muscle, and muscles depend on healthy, high-functioning mitochondria to work properly. NAD+ is a key regulator of mitochondrial function and metabolism, and it’s an important player in heart health. NAD+ can be depleted in the DNA damage response and in the body’s effort to deal with overconsumption of glucose via activation of the polyol pathway. NAD+ levels also decline with age.
The Scenarios
The above mechanisms play out in multiple, varied scenarios that, if we live long enough, will affect many of us directly, as well as our family and friends. These scenarios include:
Scenario 1: Sudden Cardiac Death and Stress-driven Magnesium Depletion
Sudden cardiac death accounts for over 50 percent of heart-related deaths. Not surprisingly, periodically, some famous person, typically male, drops dead from sudden cardiac death. These events are often described as “a massive heart attack” or cardiac arrest. The list of people smitten in their prime by sudden cardiac death include:
- James Gandolfini, actor, The Sopranos, age fifty-one.
- Tim Russert, journalist, age fifty-eight.
- Michael Jackson, king of pop, age fifty.
- Karen Carpenter, singer, age thirty-two.
- Mohamed Morsi, former Egyptian president, age sixty-seven.
The list could go on. There’s definitely something attention-grabbing about a prominent person dropping dead. It could have been us. They might have been younger, thinner, or more athletic than us, or perhaps they shared some common health challenges or bad habits with us.
While reporting on these events tends to emphasize the standard high cholesterol, clogged arteries party line, the majority of men and women who die of sudden cardiac death have no history of clinically diagnosed heart disease. A model from a redox health perspective posits that stress hormones and oxidative stress deplete intracellular magnesium levels. Magnesium plays a key role in every pathology of cardiovascular disease, but especially in the electrical function of the heart. In virtually every instance of cardiac arrest or sudden cardiac death, you can identify probable acute and chronic stressors. These, over time and in the moment, deplete both stress resilience (e.g., glutathione and NAD+) and cellular magnesium levels to the point that arrhythmias occur, and in some instances these electrical malfunctions are fatal.
While there can be other contributors to oxidative stress load and arrhythmic events, magnesium stands out for its regulatory role in electrolyte balance, and the research evidence shows that low-magnesium diets can induce arrhythmias and atrial fibrillation that are resolved by magnesium supplementation. Lastly, the knowledge that magnesium levels are depleted by stress hormones and oxidative stress makes it a prime candidate for sudden cardiac death in the face of extreme life stress events, such as the death of a spouse, divorce, job loss, retirement, or relocating.
Scenario 2: Non-Fatal Arrhythmias and Atrial Fibrillation
Note that the magnesium depletion scenario in sudden cardiac death also applies to non-fatal arrhythmias and atrial fibrillation. These are electrical malfunctions of the heart and are indicative of electrolyte imbalances.
I need to note that these electrolyte imbalances can also involve high or low calcium, magnesium, potassium, sodium, or phosphate, and that while these other imbalances are often related to low magnesium levels, pharmaceutical use, heavy exercise, diarrhea, or stress-driven activation of the HPA axis and renin–angiotensin–aldosterone system can also play a role. If your doctor has prescribed medications for blood pressure or other conditions, it’s always prudent to consult them before starting any new treatment protocol.
While stress-driven magnesium depletion doesn’t explain every arrhythmia and cardiac arrest, there is enough research evidence to suggest that it’s vastly more common than currently recognized.
Scenario 3: Heart Attack
In heart attack (myocardial infarction), redox-driven damage and dysregulation is playing out in a highly localized scenario (the heart) at a cellular and tissue level. The oxidative stressor that initiates the heart attack is typically reduced blood flow to the heart (ischemia), resulting in oxygen deprivation (hypoxia). Paradoxically, restoring that flow (reperfusion) is also an oxidative stressor. Recent research notes that the body’s effort to deal with the increased oxidative stress results in reductive stress, and both stressors negatively impact energy production (mitochondrial function) in the heart muscle. They also affect protein synthesis (endoplasmic reticulum stress), disrupt heart rhythms, and can lead to cell and tissue death.
Given the prevalence of cardiovascular disease, there are many research articles on myocardial infarction and ischemia-reperfusion injury, how they work, and how to prevent or lessen their impact. At a high level, we know that the things that enhance stress resilience are protective against heart disease. Magnesium, plant polyphenols, nicotinamide riboside (a NAD+ precursor), omega-3 fats—all reduce the size and severity of a heart attack.
Getting to the point where your arteries are damaged and inflamed enough to cut off blood flow to your heart typically takes years of sustained redox imbalance from whatever stressors are pushing your cumulative stress load to pathological levels. We also know that heart disease is reversible with a nutrient-dense, plant-based diet, so it’s not too late to make changes that could prevent the pain and cost of bypass surgery.
Scenario 4: Atherosclerosis and Arterial Stiffness
There was a time I thought your arteries progressively narrowed until you had to have them surgically replaced. That gave you another twenty years, then you died. That’s kind of how it worked for my dad. He lived to eighty-five despite having a family history of early death from heart disease, and familial hypercholesterolemia, a heritable genetic predisposition to high cholesterol. Western medicine has largely framed heart disease this way, with decades of statin use as the prophylactic to delay that inevitable quadruple bypass. It doesn’t have to be that way.
While all stressors contribute to your cumulative stress load, the ones we’re particularly interested in for atherosclerosis are the chronic lifestyle behaviors and pro-oxidant states that keep your body and specifically your circulatory system inflamed. This is the engine of atherosclerosis—injury that causes an immune response and resulting inflammation, thickening of the vessel wall, and the development of atherosclerotic lesions and plaques.
The underlying mechanisms of calcification and atherosclerosis can also affect heart valve function in conditions such as aortic valve stenosis, a narrowing of the aortic valve.
Scenario 5: Heart Failure
The heart is a muscle, and muscles rely on a well-regulated metabolism and healthy mitochondria to supply sufficient energy for proper function. In heart failure, mitochondrial function and energy production is sufficiently degraded that the pumping action of the heart is impaired.[52] At the time of publication, the standard therapy for heart failure relies almost entirely on hypertension medications—some combination of ACE inhibitors, angiotensin II receptor blockers, aldosterone antagonists, diuretics, beta blockers, calcium channel blockers, etc. These mitigate the disease symptoms, but do not explicitly address the underlying cause: redox-driven mitochondrial dysfunction.
Scientific consensus is increasing that oxidative stress causes heart failure and other heart pathologies like hypertension, atherosclerosis, heart attack, and arrhythmias. In heart failure, oxidative stress drives the metabolic and mitochondrial dysfunction behind the reduced muscle function. In the last decade, we have learned much about how to improve mitochondrial function. Among the most promising strategies:
- AMPK activation: AMP-activated protein kinase (AMPK) is an enzyme that regulates metabolism. Compounds that activate AMPK have benefits for heart disease and diabetes. The diabetes medication metformin is among the best-known AMPK activators, but there many others, including berberine, lipoic acid, quercetin, rutin, resveratrol, ginseng, and curcumin. All these compounds have research support for benefit in heart failure and strong safety profiles.
- Exercise –> mitochondrial biogenesis: Mitochondrial biogenesis is an important metabolic process where cells produce more and bigger mitochondria, enhancing energy production. While there are compounds that stimulate mitochondrial biogenesis, including resveratrol, rutin, quercetin, hydroxytyrosol (from olives/olive leaf), and green tea catechins, the most effective strategy for accomplishing this is exercise.
- Metabolic nutrients: Stress/redox stress has nutritional impacts that are well-known but almost never used in a clinical setting. Many of these affect mitochondrial function and energy production, and addressing these nutritional deficits can significantly improve heart failure symptoms and muscle function. The most studied of these include:
- Magnesium
- L-carnitine
- D-ribose
- NAD+. NAD+ is a key regulator of metabolism. When NAD+ levels are low, energy production is impaired. Research has shown that NAD+ levels can be raised by supplementing with nicotinamide riboside or nicotinamide mononucleotide
- CoQ10/ubiquinol
While I’m not specifying a protocol here, I do believe that the above components form the basis of a strategy for addressing the root cause of metabolic issues involved in heart failure.
Scenario 6: Physical Damage to the Heart and Valves
Oxidative stress can cause damage to the heart long before there are clinical signs and symptoms that lead to diagnosis and intervention. We are seeing evidence of this in post-COVID inflammatory conditions affecting the heart tissue (e.g., myocarditis). While the role of oxidative stress in COVID-19 pathologies is not widely recognized in popular coverage, there are many researchers pursuing this approach.[53] Other examples of oxidative stress-driven cardiac damage include:
- Cardiac hypertrophy and cardiac remodeling.[54] Stress and oxidative stress from various sources can lead to cardiac hypertrophy and cardiac remodeling. These potential sources include exercise, hypertension, the genetic condition Marfan syndrome, coronary valve disease, and heart attack.
- Mitral valve prolapse and regurgitation.[55] Recent studies show that the oxidative stress that accompanies mitral valve prolapse (MVP) is not resolved by surgery. This suggests that oxidative stress is a cause of MVP, rather than a result of MVP.
- Ischemia/reperfusion (I/R) injury. During hypoxia caused by ischemic blockage of blood flow, cardiac cells adapt to low oxygen conditions. When blood flow and oxygen is restored, these same cells experience a whipsaw shift to reductive stress that is often fatal. This is the core pathology of damage in heart attack.
Redox Imbalance and Heart Disease: Key Takeaways
- Oxidative stress causes the inflammation, tissue damage and systemic dysregulation that lead to atherosclerosis and ischaemic heart disease (loss of blood and oxygen flow to the heart).
- It also causes nutritional deficits for important heart-related nutrients like magnesium and vitamin D. Low magnesium status makes it impossible to maintain healthy antioxidant capacity and is involved in every pathology of heart disease including the electrical function of the heart.
- Reductive stress, specifically an imbalance of NAD+ and NADH, negatively impacts energy production for your body’s most energy-intensive organ: the heart.
- Addressing redox imbalance through a combination of a nutrient-dense diet, regular exercise, adequate sleep and intelligent supplementation should protect you from early death and delay cardiovascular disease pathologies better than any current pharmaceutical therapy.
Redox Imbalance and Heart Disease: Action Steps
- Implement the foundational redox health practices and interventions discussed in chapter 2.
- If you have elevated blood glucose (> 90 fasting) and/or high triglyceride levels (> 2:1 triglyceride/HDL ratio), they are causing oxidative stress and inflammation that contribute to atherosclerosis and heart disease. You can and should lower them by reducing intake of processed carbs and sugars.
- If you have central obesity (ie. waist/height ratio > 1–2), you are very likely in a pro-oxidant, pro-inflammatory state, thanks to white fat, which produces pro-inflammatory cytokines and chemokines. Improving your waist/height ratio will (ie. losing weight and gaining muscle mass through exercise) will lower your cumulative stress burden.
- Heart rate variability (HRV) is a useful biomarker for understanding both heart health and sympathetic/parasympathetic balance. Sympathetic dominance is a useful stand-in for redox imbalance and low heart rate variability tracks with oxidative stress. Apple Watch now measures HRV, but the gold standard is still to use a chest strap monitor (about $50) and an app like EliteHRV, which interprets your results for you. Exercise and meditation/mindfulness exercises will improve HRV scores.
- Resting heart rate (RHR) is a simple and useful biomarker for autonomic nervous system health, with elevated RHR (> 70bpm) being both an independent risk factor for heart disease and diabetes. Practicing high-intensity interval training (HIIT) lowers RHR.
- A combination of aerobic, resistance and high-intensity interval training improve vascular health and microcirculation, improve metabolism and mitochondrial function and enhance stress resilience through hormesis.
- Evidence suggests that magnesium supplementation should be a core therapy in redox imbalance. The combination of magnesium, vitamin K2 and vitamin D3 appears to be synergistic with magnesium enhancing vitamin D synthesis, and Vitamin K2 inhibiting atherosclerosis.
- Reduce the intake of grain oils to keep your omega-6/omega-3 ratio as close to 1 to 1 as possible. Flaxseed, chia seeds, walnuts, algae, seaweed and fatty fish are important food sources of omega-3 fats, with marine sources being richer in EPA and DHA forms.
- Quercetin and rutin are heart-healthy supplements that reduce inflammation and improve vascular health. Rutin, in particular, raises nitric oxide (NO) levels in your blood vessels, an important strategy for improving vascular health.
- Taurine, nicotinamide riboside and nicotinamide mononucleotide raise NAD+ and promote healthy metabolism.
- Note: talk to your doctor before starting any supplement or therapeutic intervention.
Standard caveat: I am not a doctor. This is not medical advice.